1.1 understand the three states of matter in terms of the arrangement, movement and energy of the particles
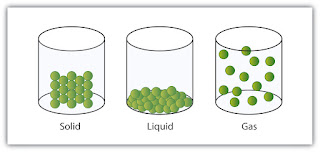
Solids
Particles touch and vibrate around a fixed point. They are in a fixed neat order. Particles have little energy.
Liquids
Particles touch. They are in a random order. Particles move over each other but are always touching at least one other particle. Particles have more energy than a solid but less than a gas.
Gas
Particles are far apart and bounce randomly around the container. Particles are not touching, though they may frequently collide. Particles have a great amount of energy.
Comments
Post a Comment